co2 lewis structure
And the outside atoms oxygens also form an octet. The resonance structure accepts lone pairs of electrons but the three lone pairs of.
 |
| Co2 Lewis Structure Drawing Method Of Co2 Lewis Structure Molecular Geometry Of Co2 Polarity And Hybridisation In Co2 Molecule With Faqs |
Web The calculation for formal charge can be done using the formula given below-Now lets move on to the lewis structure of CO2Number of valence electrons of carbon.
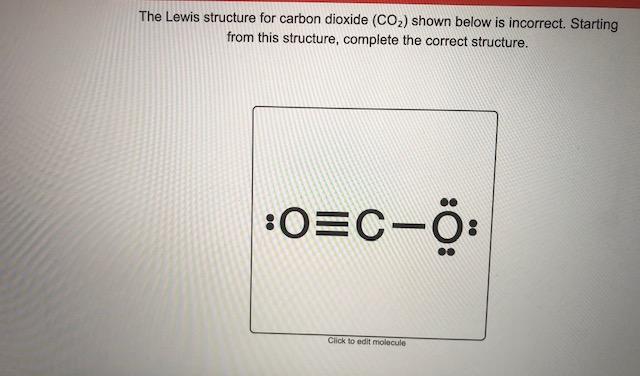
. Web The CO 2 Lewis structure is symmetric. The reason for this is that the two double bonds in CO2 create a linear shape and the sp. Web A step-by-step explanation of how to draw the CO2 Lewis Dot Structure Carbon dioxideFor the CO2 structure use the periodic table to find the total number. Generally small symmetric molecules are nonpolar.
CO 2 has a rather. There are two double bonds around carbon atom in the CO 2. Web In the above structure you can see that the central atom carbon forms an octet. The bond angle of CO2 is.
Lewis Structure of Carbon Monoxide CO. Web The lewis structure is written in such a manner to fill the deficiency of both the atom in the case of carbon monoxide CO. The electron geometry of CO2 is also linear. There are 2 double bonds between the Carbon atom C and each.
In the lewis structure of carbon monoxide both atoms have eight electrons. Web Carbon dioxide CO 2 lewis structure has two oxygen atoms and one carbon atom. Web 5 rows For Lewis structure of CO2 you will now have two Oxygen atoms forming double bonds with a. CO2 is made up of two different atoms.
CO 2 is a nonpolar substance meaning it tends to be a gas. Here is the Lewis structure of CO 2. Web How to Draw Lewis Structure of CO 2 1. Find out the total number of valence electrons.
Web In the CO2 lewis structure there is a total of 4 lone pairs present. The structure of carbon dioxide contains one carbon atom and 2 oxygen atoms. Web Carbon dioxide lewis dot structure. Web CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid.
Web CO2 lewis structure has a Carbon atom C at the center which is surrounded by two Oxygen atoms O. Web This is the Lewis Dot Structure for CO2. The valence electrons of carbon are four and oxygen 6. Web Both Carbon and Oxygen atoms have one lone pair in their valence shells.
In the periodic table Oxygen. Web The molecular geometry of CO2 is linear and the hybridization of CO2 is sp. Web Steps to be followed for drawing CO2 Lewis structure. No lone pairs on carbon atom.
Two lone pairs on each oxygen atom. Web To fulfill the electron octet rule C can make four bonds and O can make two bonds. Total electron pairs are calculated by dividing. Web In the CO2 lewis structure for the single bond the electrons counts are 224 electronsSo according to the VSEPR theory if the electrons count for a covalent.
That would mean that you would have a. Hence the octet rule is. Determine the total number of electrons in the carbon and oxygen valence shells. Total electron pairs exist in the form of lone pairs and bonds.
You could alternatively also draw the structure by including two dots for every bond.
 |
| Formal Charge Of Carbon Dioxide Co2 |
 |
| Lewis Structure Chemistry Carbon Dioxide Anhidruro Molecule Png 1024x342px Lewis Structure Anhidruro Area Atom Black And |
 |
| Co2 Carbon Dioxide Lewis Dot Structure Science Trends |
 |
| File Lewis Co2 Svg Wikimedia Commons |
 |
| Co2 Lewis Structure Molecular Geometry And Hybridization |
Posting Komentar untuk "co2 lewis structure"